 |
|
 |
|
TOTAL DISSOLVED SOLIDS (TDS)
Similar to specific conductance, the concentration of total dissolved solids (TDS) generally increases in Boulder Creek as the creek moves downstream from the mountains to the plains (for example, see
September, 2000). 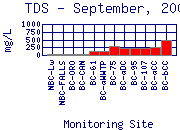 The increase in TDS is partly due to geology of the watershed, and partly due to humansí effects on the watershed. At mountain and canyon sampling sites the underlying geology is composed of granitic and metamorphic rocks, which are resistant to weathering and thus donít provide a significant amount of dissolved solids to the creek. TDS concentrations at these sites generally range from 20 to 100 mg/L.
The increase in TDS is partly due to geology of the watershed, and partly due to humansí effects on the watershed. At mountain and canyon sampling sites the underlying geology is composed of granitic and metamorphic rocks, which are resistant to weathering and thus donít provide a significant amount of dissolved solids to the creek. TDS concentrations at these sites generally range from 20 to 100 mg/L.
![]() At the plains sites, the underlying geology consists of less resistant sedimentary rocks, which release more dissolved solids. These sites are also subjected to more human influences, such as road runoff and fertilizer runoff. After passing through the City of Boulder, TDS concentrations are typically between 40 and 200 mg/L.
At the plains sites, the underlying geology consists of less resistant sedimentary rocks, which release more dissolved solids. These sites are also subjected to more human influences, such as road runoff and fertilizer runoff. After passing through the City of Boulder, TDS concentrations are typically between 40 and 200 mg/L.
At 75th Street, just downstream from the City of Boulderís Wastewater Treatment Plant (WWTP), TDS concentrations increase significantly in Boulder Creek to values between 240 and 420 mg/L. This increase is due to effluent discharged from the WWTP. While the wastewater from Boulder residences, businesses, and industries is treated to remove most constituents and meets State of Colorado and U.S. Environmental Protection Agency (EPA) requirements, it is impossible for the plant to remove all of the dissolved solids contributed to the sanitary sewer by all the homes and businesses in Boulder.
Downstream from 75th Street, TDS concentrations of Boulder Creek typically remain fairly constant to the confluence with Coal Creek. During some times of the year, TDS concentrations decrease downstream of the WWTP, because of dilution; at the others of the year, TDS concentrations increase, indicating a source of dissolved constituents to the Creek.
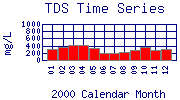 TDS concentrations at each site vary seasonally, due to the highly variable flow rate of Boulder Creek. During high-flow months, such as May, June, and August, TDS concentrations are lower than during low-flow months, because the greater volume of water dilutes ion concentrations. TDS concentrations tend to be highest in March or April; this may be due to flushing of forest litter and soils as snow begins to melt. For example, see
BC-75 in 2000.
TDS concentrations at each site vary seasonally, due to the highly variable flow rate of Boulder Creek. During high-flow months, such as May, June, and August, TDS concentrations are lower than during low-flow months, because the greater volume of water dilutes ion concentrations. TDS concentrations tend to be highest in March or April; this may be due to flushing of forest litter and soils as snow begins to melt. For example, see
BC-75 in 2000.

COMPARISON TO REGULATIONS
Other than U.S. Environmental Protection Agency (EPA) secondary standards for drinking water (500 mg/L), there are no water quality standards for TDS. However, high concentrations of TDS are harmful to aquatic life. For comparison, unpolluted rainwater has a TDS approaching 0 mg/L, while seawater contains about 36,000 mg/L.