 |
|
 |
|
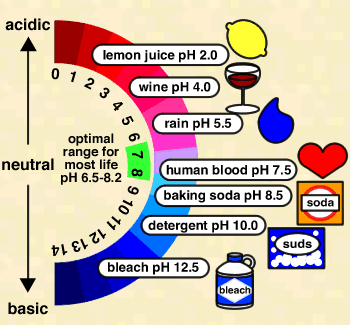
pH
pH = -log [H+]
where [H+] is the concentration of H+ ions in moles per liter (a mole is a unit of measurement, equal to 6.022 x 1023 atoms). Because H+ ions associate with water molecules to form hydronium (H3O+) ions, pH is often expressed in terms of the concentration of hydronium ions. In pure water at 22° C (72° F), H3O+ and hydroxyl (OH-) ions exist in equal quantities; the concentration of each is 1.0 x 10-7 moles per liter (mol/L). Therefore, pH of pure water = -log (1.0 x 10-7) = -(-7.00) = 7.00. Because pH is defined as –log [H+], pH decreases as [H+] increases (which will happen if acid is added to the water). Since pH is a log scale based on 10, the pH changes by 1 for every power of 10 change in [H+]. A solution of pH 3 has an H+ concentration 10 times that of a solution of pH 4. The pH scale ranges from 0 to 14. However, pH values less than 0 and greater than 14 have been observed in very rare concentrated solutions.
The pH of water can be measured with a pH meter, which is an electronic device
with a probe. The probe contains an acidic aqueous solution enclosed by a glass
 membrane that allows migration of H+ ions. The electrical potential
of the glass electrode depends on the difference in [H+] between
the reference solution and the solution into which the electrode is dipped.
pH can also be measured with pH paper or by adding a reagent (indicator solution)
to the water sample and recording the color change.
membrane that allows migration of H+ ions. The electrical potential
of the glass electrode depends on the difference in [H+] between
the reference solution and the solution into which the electrode is dipped.
pH can also be measured with pH paper or by adding a reagent (indicator solution)
to the water sample and recording the color change.
The concentration of carbon dioxide in the water
Carbon dioxide (CO2) enters a water body from a variety of sources, including the atmosphere, runoff from land, release from bacteria in the water, and respiration by aquatic organisms. This dissolved CO2 forms a weak acid. Natural, unpolluted rainwater can be as acidic as pH 5.6, because it absorbs CO2 as it falls through the air. Because plants take in CO2 during the day and release it during the night, pH levels in water can change from daytime to night. For an example of how pH typically varies over a daily cycle, select here.
Geology and Soils of the watershed

Acidic and alkaline compounds can be released into water from different types of rock and soil. When calcite (CaCO3) is present, carbonates (HCO3, CO3-2) can be released, increasing the alkalinity of the water, which raises the pH. When sulfide minerals, such as pyrite, or "fool’s gold," (FeS2) are present, water and oxygen interact with the minerals to form sulfuric acid (H2SO4). This can significantly drop the pH of the water. Drainage water from forests and marshes is often slightly acidic, due to the presence of organic acids produced by decaying vegetation.
Drainage from Mine Sites
Mining for gold, silver, and other metals often involves the removal of sulfide minerals buried in the ground. When water flows over or through sulfidic waste rock or tailings exposed at a mine site, this water can become acidic from the formation of sulfuric acid. In the absence of buffering material, such as calcareous rocks, streams that receive drainage from mine sites can have low pH levels.
Air Pollution 
Air pollution from car exhaust and power plant emissions increases the concentrations of nitrogen oxides (NO2, NO3) and sulfur dioxide (SO2) in the air. These pollutants can travel far from their place of origin, and react in the atmosphere to form nitric acid (HNO3) and sulfuric acid (H2SO4). These acids can affect the pH of streams by combining with moisture in the air and falling to the earth as acid rain or snow.

Water Quality Standards and Other Criteria Regarding pH
The U.S. Environmental Protection Agency (U.S. EPA) sets a secondary standard for pH levels in drinking water: the water should be between pH 6.5 and 8.5 Secondary standards are unenforceable, but recommended, guidelines.
Colorado Department of Public Health and Environment Water Quality Control Division (CDPHE-WQCD) regulations (5 CCR 1002-31) state that waters to be used for domestic water supply should have pH values between 5.0 and 9.0 (Reg. 31 - Basic Standards and Methodologies for Surface Water).
 CDPHE-WQCD regulations state that waters used for primary recreation (including such activities as swimming, rafting, and kayaking) should have pH values between 6.5 and 9.0.
CDPHE-WQCD regulations state that waters used for primary recreation (including such activities as swimming, rafting, and kayaking) should have pH values between 6.5 and 9.0.
Very high ( greater than 9.5) or very low (less than 4.5) pH values are unsuitable for most aquatic organisms. Young fish and immature stages of aquatic insects are extremely sensitive to pH levels below 5 and may die at these low pH values. High pH levels (9-14) can harm fish by denaturing cellular membranes.
Changes in pH can also affect aquatic life indirectly by altering other aspects of water chemistry. Low pH levels accelerate the release of metals from rocks or sediments in the stream. These metals can affect a fish’s metabolism and the fish’s ability to take water in through the gills, and can kill fish fry.
Other Information about pH
The term "pH" was originally derived from the French term "pouvoir hydrogène," in English, this means "hydrogen power." The term pH is always written with a lower case p and an upper case H.