 |
|
 |
|
pH
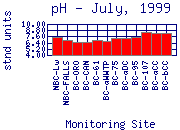 In general, the pH of Boulder Creek increases as it moves downstream
(for example July, 1999). The pH of mountain sampling sites is typically between 6.5 and 8; the pH
through the City of Boulder is typically between 6.8 and 8.8; the pH in lower Boulder
Creek is typically between 7.3 and 10.
In general, the pH of Boulder Creek increases as it moves downstream
(for example July, 1999). The pH of mountain sampling sites is typically between 6.5 and 8; the pH
through the City of Boulder is typically between 6.8 and 8.8; the pH in lower Boulder
Creek is typically between 7.3 and 10.
![]() This increase is due in part to changes in geology. In the mountains, Boulder Creek passes over granitic and metamorphic
rocks, which provide little to no buffering capacity to the water. As Boulder
Creek moves into the plains, the geology changes to sedimentary rocks,
including sandstones and calcareous (calcite-containing) shales. The calcite
(CaCO3) dissolves in the water, and hydrogen ions are consumed in
this reaction. This loss of hydrogen ions from the water increases the pH of
the stream.
This increase is due in part to changes in geology. In the mountains, Boulder Creek passes over granitic and metamorphic
rocks, which provide little to no buffering capacity to the water. As Boulder
Creek moves into the plains, the geology changes to sedimentary rocks,
including sandstones and calcareous (calcite-containing) shales. The calcite
(CaCO3) dissolves in the water, and hydrogen ions are consumed in
this reaction. This loss of hydrogen ions from the water increases the pH of
the stream.
The pH of Boulder Creek drops at 75th Street. This drop is due to the effluent from Boulder's Wastewater Treatment Plant (WWTP), which has a lower pH than the water in the creek.
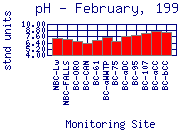
pH levels can be very high at the sampling sites downstream of the WWTP.
While lower Boulder Creek contains more alkalinity than sampling sites in the
mountains, alkalinity levels remains relatively low. Therefore, the pH is not
very well buffered and can be easily altered by other factors. The pH in lower
Boulder Creek shows extreme diurnal (day to night) variation for the same
reason as does dissolved oxygen (DO) concentration: photosynthetic activity. Nutrients, released
from the WWTP and from fertilizer runoff, feed aquatic plants and algae in the
creek and allow significant plant growth to occur. The lack of riparian
vegetation along much of Lower Boulder Creek allows significant sunlight to
reach the creek, which further increases plant growth.
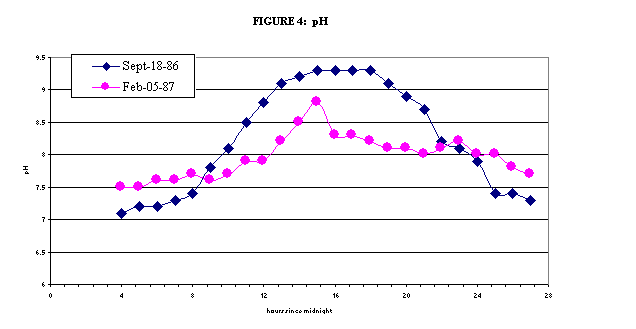 During the day, plants
consume carbon dioxide (CO2) while undergoing photosynthesis. The
removal of CO2 from the water causes bicarbonate and carbonate ions
to react with hydrogen ions (H+) to form more CO2. This
loss of H+ from the water causes the pH to increase. At night
time, when plants, bacteria, and animals are undergoing respiration,
CO2 is released and oxygen is consumed. This releases
H+ back to the water. Therefore, pH values during the day can be
much higher than during the night at these sites. Monthly water samples are
collected during the day; therefore, the samples from lower Boulder Creek
reflect the high pH values. As with DO levels, the magnitude of the
difference from night and day is affected by the season. During the summer,
plants are exposed to sunlight for a much longer time, so they are growing
faster, and the pH levels show the greatest variation at this time. More information on the daily variation of pH downstream of the WWTP can be found here.
During the day, plants
consume carbon dioxide (CO2) while undergoing photosynthesis. The
removal of CO2 from the water causes bicarbonate and carbonate ions
to react with hydrogen ions (H+) to form more CO2. This
loss of H+ from the water causes the pH to increase. At night
time, when plants, bacteria, and animals are undergoing respiration,
CO2 is released and oxygen is consumed. This releases
H+ back to the water. Therefore, pH values during the day can be
much higher than during the night at these sites. Monthly water samples are
collected during the day; therefore, the samples from lower Boulder Creek
reflect the high pH values. As with DO levels, the magnitude of the
difference from night and day is affected by the season. During the summer,
plants are exposed to sunlight for a much longer time, so they are growing
faster, and the pH levels show the greatest variation at this time. More information on the daily variation of pH downstream of the WWTP can be found here.
COMPARISON TO REGULATIONS
Colorado Department of Public Health and Environment Water Quality Control Division (CDPHE-WQCD) regulations state that waters used for primary recreation (including such activities as swimming, rafting, and kayaking), waters classified as "Class 1 Cold Water Aquatic Life," and waters classified as "Class 1 Warm Water Aquatic Life" should have pH values between 6.5 and 9.0. Most of the samples collected by the City of Boulder from Boulder Creek had pH values between 6.5 and 9.0 during 1998 to 2000. However, some of the samples from lower Boulder Creek - including Boulder Creek at Highway 287 (BC-107), Boulder Creek above the confluence with Coal Creek, (BC-aCC), and Boulder Creek above the confluence with Coal Creek (BC-bCC) - had pH values greater than 9.0 during hot summer months (for example July, 1998). These pH values are not healthy for aquatic organisms.
All source water samples collected in 1998 and 2001 from North Boulder Creek and Middle Boulder Creek (used for Boulder domestic water supply) had pH values between 5.0 and 9.0, in accordance with the Colorado Department of Public Health and Environment Water Quality Control Division (CDPHE-WQCD) requirement for waters to be used for domestic water supply.