
|
Map bar plots are plotted on the following scale 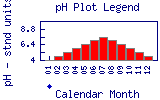  indicates no data available. indicates no data available. | |
Boulder Reservoir pH Time Series for 1999 |

|
Map bar plots are plotted on the following scale   indicates no data available. indicates no data available. | |
Boulder Reservoir pH Time Series for 1999 |
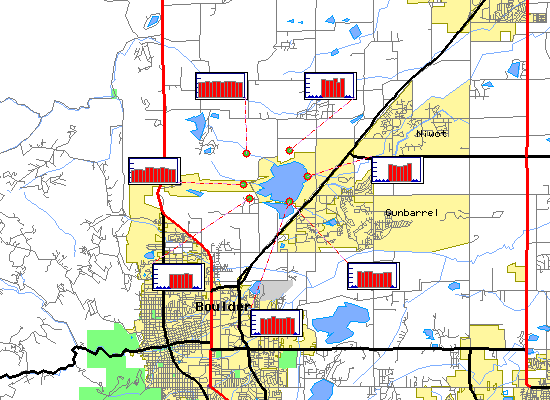
Select on the map plot to view detailed time series information for pH at that site. Select on the site icon to view time series information for all parameters at that site.
pH measures hydrogen concentration in water. pH is presented on a scale from 0 to 14. A solution with a pH value of 7 is neutral; a solution with a pH value less than 7 is acidic; a solution with a pH value greater than 7 is basic. Natural waters usually have a pH between 6 and 9. The scale is negatively logarithmic, so each whole number (reading downward) is ten times the preceding one (for example, pH 5.5 is 100 times as acidic as pH 7.5). The pH of natural waters can be made acidic or basic by human activities. For example, coal-burning power plants and heavy automobile traffic can produce nitrous oxides and sulfur dioxide, making the rain and snow highly acidic. | |||||||||
| |||||||||
|
About BASIN | Attribution | Feedback | Search | |||||