|
|

|
City of Boulder Storm Water Quality Program |
|
Boulder Creek Watershed
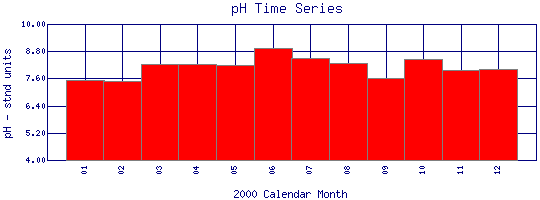
pH Time Series for 2000
Boulder Creek at 95th St |
Preliminary Data - subject to revision
|
|
|
 |
 Indicates no data available Indicates no data available |
 Indicates data exceeds plot scale. Indicates data exceeds plot scale. |
|
|
Information on pH:
pH
measures hydrogen concentration in water. pH is presented on a scale from 0 to 14. A solution with a pH value of 7 is neutral; a solution with a pH value less than 7 is acidic; a solution with a pH value greater than 7 is basic. Natural waters usually have a pH between 6 and 9. The scale is negatively logarithmic, so each whole number (reading downward) is ten times the preceding one (for example, pH 5.5 is 100 times as acidic as pH 7.5). The pH of natural waters can be made acidic or basic by human activities. For example, coal-burning power plants and heavy automobile traffic can produce nitrous oxides and sulfur dioxide, making the rain and snow highly acidic. |
|
More general information about pH
|
|
|
Interpretation of Boulder Creek pH data
|
|
|
|
Monitoring site information: |
|

|
- Location: Boulder Creek at 95th St
- Name: BC-95
- Type: Composite
- Frequency: Monthly
- Longitude: 105 ° W 78'
- Latitude: 40 ° N 28'
- Elevation: 5050 feet asl
- Site photo
|
|
| Downstream Site: Boulder Creek at 107th St |
| Upstream Site: Boulder Creek above Dry Creek |
|
| Plot Label |
Date |
pH
stnd units |
| 01 |
January, 2000 |
7.51 |
| 02 |
February, 2000 |
7.50 |
| 03 |
March, 2000 |
8.25 |
| 04 |
April, 2000 |
8.25 |
| 05 |
May, 2000 |
8.19 |
| 06 |
June, 2000 |
8.92 |
| 07 |
July, 2000 |
8.48 |
| 08 |
August, 2000 |
8.30 |
| 09 |
September, 2000 |
7.60 |
| 10 |
October, 2000 |
8.46 |
| 11 |
November, 2000 |
7.95 |
| 12 |
December, 2000 |
8.01 |
| *value below detection limit: |
|---|
| Detection Limit (if available) |
NA |
|---|
|
Preliminary Data - subject to revision
|
|
Select on the parameter name to view all pH time series plots on the basin map.
Select on the table date to view stream pH profiles observed on that date.
Select here to view all parameters at this site.
|
INVITATION:
BASIN is a community project actively seeking public participation. We
appreciate all feedback and welcome comments, suggestions and contributions.
To find out more about how you can be involved,
click here.
|
|
Home
| Site Map
| Glossary
| Bibliography
| Contributors
About BASIN
| Attribution
| Feedback
| Search
|

 Indicates no data available
Indicates no data available Indicates data exceeds plot scale.
Indicates data exceeds plot scale.

