 |
|
 |
|
Nitrogen is required by all organisms for the basic processes of life to make proteins, to grow, and to reproduce. Nitrogen is very common and found in many forms in the environment. Inorganic forms include nitrate (NO3), nitrite (NO2), ammonia (NH3), and nitrogen gas (N2). Organic nitrogen is found in the cells of all living things and is a component of proteins, peptides, and amino acids. Nitrogen is most abundant in Earth’s environment as N2 gas, which makes up about 78 percent of the air we breathe.
The Nitrogen Cycle
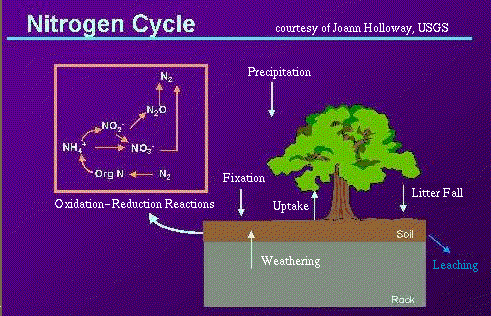
Nitrogen is recycled continually by plants and animals. This recycling of nitrogen through the environment is called the "nitrogen cycle."
Most organisms (including humans) can't use nitrogen in the gaseous form N2 for their nutrition, so they are dependent on other organisms to convert nitrogen gas to nitrate, ammonia, or amino acids. "Fixation" is the conversion of gaseous nitrogen to ammonia or nitrate. The most common kind of fixation is "biological fixation" which is carried out by a variety of organisms, including blue-green algae, the soil bacteria Azobacter, and the association of legume plants and the bacteria Rhizobium. Additionally, nitrogen can be fixed by some inorganic processes. For example, "high-energy fixation" occurs in the atmosphere as a result of lightning, cosmic radiation, and meteorite trails. Atmospheric nitrogen and oxygen combine to form nitrous oxides (NOx), which fall to the earth as nitrate.
When plants and animals die, proteins (which contain organic nitrogen) are broken down by bacteria to form ammonia (NH3). This process is called "ammonification." Ammonia is then broken down by other bacteria (Nitrosomonas) to form nitrite (NO2), which is then broken down by another type of bacteria (Nitrobacter) to form nitrate (NO3). This conversion of ammonia to nitrate and nitrite is called "nitrification." Nitrates can then be used by plants in order to grow.
Completing the nitrogen cycle, nitrates are reduced to gaseous nitrogen by the process of "denitrification." This process is performed by organisms such as fungi and the bacteria Pseudomonas. These organisms break down nitrates to obtain oxygen.
Common Forms of Nitrogen in Water
Nitrate and Nitrite
Nitrate (NO3) is highly soluble (dissolves easily) in water and is stable over a wide range of environmental conditions. It is easily transported in streams and groundwater. Nitrates feed plankton (microscopic plants and animals that live in water), aquatic plants, and algae, which are then eaten by fish. Nitrite (NO2) is relatively short-lived in water because it is quickly converted to nitrate by bacteria.
Excessive concentrations of nitrate and/or nitrite can be harmful to humans and wildlife. Nitrate is of most concern for humans. Nitrate is broken down in our intestines to become nitrite. Nitrite reacts with hemoglobin in human blood to produce methemoglobin, which limits the ability of red blood cells to carry oxygen. This condition is called methemoglobinemia or "blue baby" syndrome (because the nose and tips of ears can appear blue from lack of oxygen). It is especially serious for infants, because they lack the enzyme necessary to correct this condition. Wells contaminated by sewage or agricultural runoff are a major concern in some areas, because of the possibility of water high in nitrite/nitrates and the subsequent increased risk of blue baby disease. High nitrate and nitrite levels can also cause methemoglobinemia in livestock and other animals.
High concentrations of nitrate and/or nitrite can produce "brown blood disease" in fish. Nitrite enters the bloodstream through the gills and turns the blood a chocolate-brown color. As in humans, nitrite reacts with hemoglobin to form methemoglobin. Brown blood cannot carry sufficient amounts of oxygen, and affected fish can suffocate despite adequate oxygen concentration in the water. This accounts for the gasping behavior often observed in fish with brown blood disease, even when oxygen levels are relatively high (Mississippi State University, 1998).
If excessive amounts of phosphorus and nitrates are added to the water, algae and aquatic plants can be produced in large quantities. When these algae die, bacteria decompose them, and use up oxygen. This process is called eutrophication. Dissolved oxygen concentrations can drop too low for fish to breathe, leading to fish kills.
Ammonia
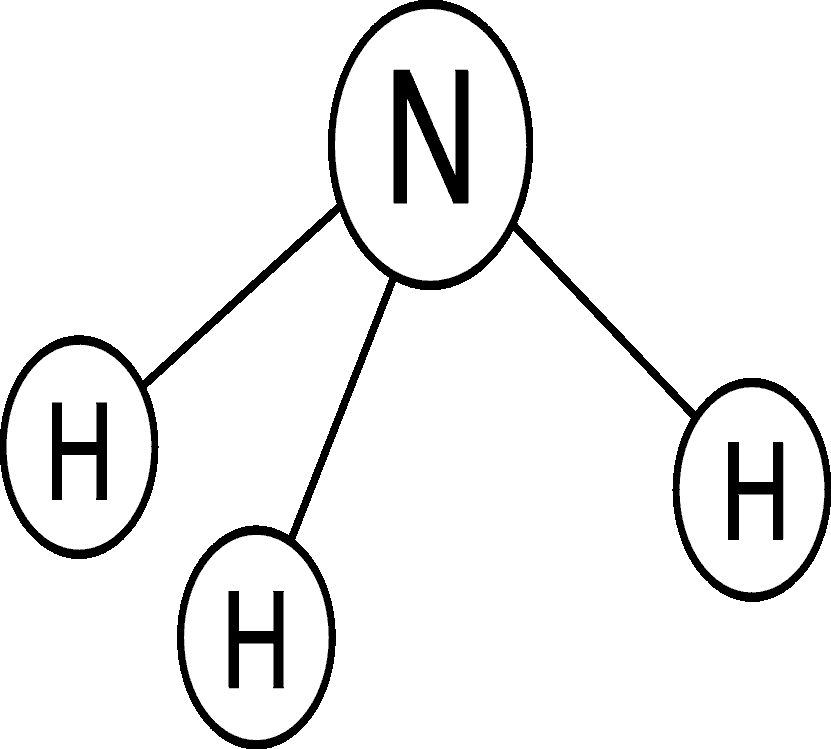
Ammonia, another inorganic form of nitrogen, is the least stable form of nitrogen in water. Ammonia is easily transformed to nitrate in waters that contain oxygen and can be transformed to nitrogen gas in waters that are low in oxygen. Ammonia is found in water in two forms - the ammonium ion (NH4+), and dissolved, unionized (no electrical charge) ammonia gas (NH3). Total ammonia is the sum of ammonium and unionized ammonia. The dominant form depends on the pH and temperature of the water. The reaction between the two forms is shown by this equation:
NH3 + H2O « NH4+ + OH-
The form of ammonia changes easily when pH changes. As pH increases, H+ concentration decreases, and OH- concentrations increase. This makes the equation above move left, increasing the amount of aqueous NH3. When the pH is below 8.75, NH4+ predominates. At pH 9.24, about half of aqueous NH3 is transformed to NH4+. Above pH 9.75, NH3 predominates (Hem, 1985). Unionized ammonia (NH3) is much more toxic to aquatic organisms than the ammonium ion (NH4+).
Toxic concentrations of ammonia in humans may cause loss of equilibrium, convulsions, coma, and death. Ammonia concentrations can affect hatching and growth rates of fish; changes in tissues of gills, liver, and kidneys may occur during structural development.
 There are many ways of measuring nitrogen forms. Total nitrogen
can be determined by adding chemicals to convert all of the nitrogen forms in
a sample to nitrate, and then measuring nitrate concentration. Nitrate and
nitrite can be measured together or separately. Nitrate and nitrite are most
often measured using a colorimetric method, which means the color of treated
sample reflects the concentration of the parameter. A chemical is added to
the water sample, and the darker the color of the sample, the more nitrate
and/or nitrite present. This test can be done visually, comparing the
treated sample to a set of reference colors. However, it is more accurate to
use an electronic colorimeter, which uses a light source and a photodetector
to find the concentration based on how much light is absorbed by the sample.
If nitrate and nitrite are reported separately, concentrations are given as
nitrite as nitrogen (NO2-N) and nitrate as nitrogen
(NO3-N). If they are reported together, concentrations are given
as nitrite plus nitrate as nitrogen (NO2 + NO3 -N).
Nitrate and nitrite can be measured in the field using a portable
colorimeter, such as a Hach© kit.
There are many ways of measuring nitrogen forms. Total nitrogen
can be determined by adding chemicals to convert all of the nitrogen forms in
a sample to nitrate, and then measuring nitrate concentration. Nitrate and
nitrite can be measured together or separately. Nitrate and nitrite are most
often measured using a colorimetric method, which means the color of treated
sample reflects the concentration of the parameter. A chemical is added to
the water sample, and the darker the color of the sample, the more nitrate
and/or nitrite present. This test can be done visually, comparing the
treated sample to a set of reference colors. However, it is more accurate to
use an electronic colorimeter, which uses a light source and a photodetector
to find the concentration based on how much light is absorbed by the sample.
If nitrate and nitrite are reported separately, concentrations are given as
nitrite as nitrogen (NO2-N) and nitrate as nitrogen
(NO3-N). If they are reported together, concentrations are given
as nitrite plus nitrate as nitrogen (NO2 + NO3 -N).
Nitrate and nitrite can be measured in the field using a portable
colorimeter, such as a Hach© kit.
Total ammonia (ammonium ion (NH4+) plus unionized ammonia gas (NH3)) is often measured in a laboratory by titration. Ammonia and organic nitrogen compounds are separated by distillation, then an acid (the titrant) is added to a volume of the ammonia portion. The volume of acid required to change the color of the sample reflects the ammonia concentration of the sample. The more acid needed, the more ammonia in the sample. Ammonia is the least stable form of nitrogen, so it can be difficult to measure accurately. The proportion of unionized ammonia can be calculated, using formulas that contain factors for pH and temperature.
Factors Affecting Nitrate+Nitrite Concentrations
Wastewater and Septic System Effluent
Human waste is significant contributor of nitrogen to water. Ammonia, nitrite, and nitrate are decomposition products from urea and protein, which are in human waste. Ammonia is an ingredient in many household cleaning products and is sometimes used to remove carbonate from hard water. Therefore, these nitrogen species go down the drains in our houses and businesses, and can enter streams from wastewater treatment plant (WWTPs) effluent, illegal sanitary sewer connections, and poorly functioning septic systems.
Nutrients in sewage effluent have been among the primary targets of pollution-control legislation, beginning with the Clean Water Act in 1972. Organic forms of nitrogen have largely been controlled by upgrading treatment plants, and advanced treatment processes have been used to decrease ammonia discharge. However, these processes result in an increase in nitrate discharge, so the total nitrogen discharge does not change. Therefore, concerns about fish toxicity have decreased, but the potential for eutrophication has not changed (Mueller and Helsel, 1999).
Fertilizer Runoff

Fertilizer is a major influence on nitrogen concentrations in the environment. Commercial nitrogen fertilizers are applied either as ammonia or nitrate, but ammonia is rapidly converted to nitrate in the soil. Animal manure is also used as a nitrogen fertilizer in some areas. Organic nitrogen and urea in the manure are converted to ammonia and, ultimately, to nitrate in the soil. Nitrate that is not used by plants washes from farmlands and residential and commercial lawns into storm drains and nearby streams, or seeps into groundwater.
Animal Waste

A significant amount of nitrogen is released in the wastes produced by animals. This can be a serious problem in waters near cattle feedlots, hog farms, dairies, and barnyards. Ducks and geese contribute a heavy load of nitrogen if they are present in large numbers. Excretions of aquatic organisms are very rich in ammonia, a decay product of animal proteins, but the amount of nitrogen they add to waters is usually small. Through the process of nitrification, ammonia is oxidized to nitrite and then to nitrate in water.
Fossil Fuels

The burning of fossil fuels such as gasoline and coal in cars, trucks, and power plants produces many by-products. Coal and petroleum generally contain about 1 percent nitrogen (Hem, 1985). Part of the nitrogen is converted to the gas nitric oxide (NO) during the burning of the fuel. Nitric oxide is converted by sunlight and photochemical processes in air to nitrogen oxide gases (NO and NO2, which are commonly referred together as NOx), which are a major component of smog. Nitrogen oxide gases are a major contributor to acid rain.
Industrial Discharge
Many industries use nitrogen during processing. Nitrite is sometimes used as a corrosion inhibitor in industrial process water. Ammonia is used in the production of nitric acid, urea and other nitrogen compounds, and in the production of ice and in refrigerating plants. Ammonia is also used in cleaning supplies and to remove carbonate from hard water. Water from industries is usually discharged to a wastewater treatment plant (WWTP), and may end up in a downstream water body if not completely removed in the WWTP.
Water Quality Standards and Other Criteria Regarding Nitrogen

Nitrate and Nitrite
 The U.S. Environmental Protection Agency (EPA) has established a maximum contaminant level (MCL) of 10 milligram per liter (mg/L) for nitrate as nitrogen (NO3-N) and a MCL of 1 mg/L for nitrite as nitrogen (NO2-N) in drinking water ( U.S. EPA Office of Water, Drinking Water and Health Advisories).
The U.S. Environmental Protection Agency (EPA) has established a maximum contaminant level (MCL) of 10 milligram per liter (mg/L) for nitrate as nitrogen (NO3-N) and a MCL of 1 mg/L for nitrite as nitrogen (NO2-N) in drinking water ( U.S. EPA Office of Water, Drinking Water and Health Advisories).
Colorado Department of Public Health and Environment Water Quality Control Division (CDPHE-WQCD) regulations (5 CCR 1002-31) state that for domestic water supply, the combined total of nitrite and nitrate shall not exceed 10 mg/L as N, and nitrite concentration shall not exceed 1 mg/L as N at the point of intake.
CDPHE-WQCD regulations for aquatic life do not give a maximum concentration for nitrate. Maximum concentrations of nitrite allowed for Class 1 Aquatic Life water depend on whether salmonids (such as trout) and other sensitive fish species are present and on chloride concentration in the water. See the CDPHE regulations for more information.
![]() CDPHE-WQCD regulations state that for agriculture water, the combined total of nitrite and nitrate shall not exceed 100 mg/L (as N), and the nitrite concentration shall not exceed 10 mg/L (as N).
CDPHE-WQCD regulations state that for agriculture water, the combined total of nitrite and nitrate shall not exceed 100 mg/L (as N), and the nitrite concentration shall not exceed 10 mg/L (as N).
Ammonia
 CDPHE-WQCD regulations state that for domestic water supply, chronic total ammonia concentrations shall not exceed 0.5 mg/L as N at the point of intake (that is, ammonia concentrations should not exceed 0.5 mg/L constantly over a 30-day period at the location where water enters standard treatment for drinking water).
CDPHE-WQCD regulations state that for domestic water supply, chronic total ammonia concentrations shall not exceed 0.5 mg/L as N at the point of intake (that is, ammonia concentrations should not exceed 0.5 mg/L constantly over a 30-day period at the location where water enters standard treatment for drinking water).
![]() CDPHE-WQCD regulations for Aquatic Life are based on unionized (no electrical charge) ammonia (NH3). However, monitoring programs typically measure total ammonia (which includes unionized ammonia and the ionized ammonium ion (NH4+)). There are no aquatic life regulations for total ammonia because the ionized form, which usually comprises most of the total ammonia, is considered nontoxic. The percentage of unionized ammonia can be calculated from the total ammonia concentration on a case by case basis, with a formula that factors in temperature and pH of the water being analyzed.
CDPHE-WQCD regulations for Aquatic Life are based on unionized (no electrical charge) ammonia (NH3). However, monitoring programs typically measure total ammonia (which includes unionized ammonia and the ionized ammonium ion (NH4+)). There are no aquatic life regulations for total ammonia because the ionized form, which usually comprises most of the total ammonia, is considered nontoxic. The percentage of unionized ammonia can be calculated from the total ammonia concentration on a case by case basis, with a formula that factors in temperature and pH of the water being analyzed.
CDPHE regulations state that waters classified as "Class 1 Cold Water Aquatic Life" (such as Boulder Creek from the headwaters to the confluence of South Boulder Creek with Boulder Creek) or "Class 2 Cold Water Aquatic Life" should not have chronic unionized ammonia concentrations above 0.02 mg/L as N. ("Chronic" means the level not to be exceeded by the concentration for either a single representative sample or calculated as an average of all samples collected during a thirty-day period). Waters classified as "Class 1 Warm Water Aquatic Life" (such as Boulder Creek from the confluence of South Boulder Creek with Boulder Creek to the confluence of Coal Creek with Boulder Creek) should not have chronic unionized ammonia concentrations above 0.06 mg/L as N. For waters classified as "Class 2 Warm Water Aquatic Life," the maximum allowed chronic unionized ammonia concentration will be in the 0.06 to 0.10 mg/L (as N) range, depending on aquatic life present and on other water quality factors.
CDPHE-WQCD regulations state that acute maximum unionized ammonia concentrations allowed for aquatic life depend on the class of water, the presence of cold water species, and on temperature and pH of the water. ("Acute" means the level not to be exceeded by the concentration in a single sample or calculated as an average of all samples collected during a one-day period.)
For more information, see CDPHE-WQCD regulations